From CNN Health, information about the MMRV vaccine.
May all babies be born into loving hands...
K. Michelle Doyle, CNM, NYS LM
www.localcaremidwifery.comwww.
localcaremidwifery.blogspot.com
June 28th, 2010
09:38 AM ET
By Miriam Falco
CNN Medical News Managing Editor
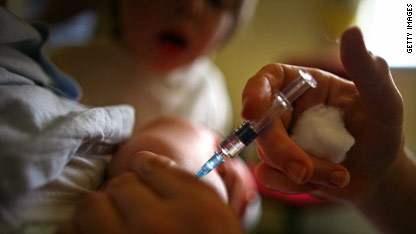
Children who get a combination of measles, mumps, rubella and chickenpox vaccines in one shot are at a slightly increased risk of getting a fever-related seizure, compared with children getting two separate shots – one containing measles, mumps and rubella and the another containing the chickenpox (varicella) vaccine, according to a new study published in the journal Pediatrics.
"The risk of a febrile seizure after any measles-containing vaccine is low – about one febrile seizure in 1,000 doses" says lead study author, Dr. Nicola Klein, co-director of Kaiser Permanente's Vaccine Study Center. "But if a child gets the combination vaccine, the risk doubles," says Klein.
Researchers looked at vaccine-safety data from more than 459,000 toddlers between the ages of 12 and 23 months and found there was one additional case of febrile seizure for every 2,300 doses of MMRV (measles, mumps, rubella, varicella) vaccine given. The seizures occurred seven to 10 days after the injection.
According to the Centers for Disease Control and Prevention, a febrile seizure is a fever-related seizure, which can occur when a child has a fever at or above 102°F or when a high fever is going down."Febrile seizures are benign," Klein says, meaning they're generally not dangerous. "They are very frightening to parents, but do not lead to long-term seizures or epilepsy." The American Academy of Pediatrics agrees: "While febrile seizures may be very scary, they are harmless to the child. Febrile seizures do not cause brain damage, nervous system problems, paralysis, mental retardation, or death."
Febrile seizures can occur in children ages 6 months to 5 years, but are most common in toddlers ages 12 months to 18 months, according to the American Academy of Family Physicians. Klein says up to 5 percent of children will have a febrile seizure between 6 months and 5 years, but that they are more likely to be caused by a common cold or other infections.
This combination vaccine was first approved in 2005. "The benefit of the MMRV is ease of administration," says Dr. William Schaffner, chairman, Department of Preventive Medicine at Vanderbilt University and a liaison to the Advisory Committee on Immunization Practices, the CDC's vaccine advisory board.
It was that ease – a single shot for four vaccines, which led the ACIP to recommend a preference for this new vaccine back in 2006, says Schaffner.
Klein presented early research to the ACIP in 2008 suggesting an increased risk of seizures. This led to the CDC to change its recommendation last year from preferring the combination vaccine to having no preference. This means MMRV or the MMR plus chickenpox vaccine may be given for the first dose for children 12-23 months.
This new study confirms Klein's earlier research. This "final result is exactly what we expected," says Schaffner. "This study provides a basis for every pediatrician."
""The ACIP has quite clearly said both [MMRV and MMR plus chickenpox separately] are good – both provide protection." But Schaffner says if there is any doubt for the parents or pediatrician, "two [vaccinations] is the way to go."
Klein says the benefit of the MMRV is "one less injection for children." Her study concludes that pediatricians who choose to use this combination vaccine need to be aware of the risks, albeit small, and clearly communicate them to parents. If parents choose to go with separate vaccinations, their child will get the MMR vaccine in one arm and the chickenpox vaccine in the other arm.
Although the MMRV has been available for five years now, usage of this combination vaccine dropped significantly after the manufacturer, Merck, announced it would be unavailable after July 2007 because of a shortage of the chickenpox vaccine.
According to the CDC, Merck was taking orders for the MMRV vaccine starting on May 10 of this year and according to the manufacturer, the vaccine is now available again.
The updated package insert for the MMRV vaccine say it "is associated with higher rates of fever and febrile seizures at 5 to 12 days after vaccination," compared with children who got the MMR and chickenpox shots separately.
No comments:
Post a Comment